RADIATION BIOLOGY
ORBITING ELECTRONS
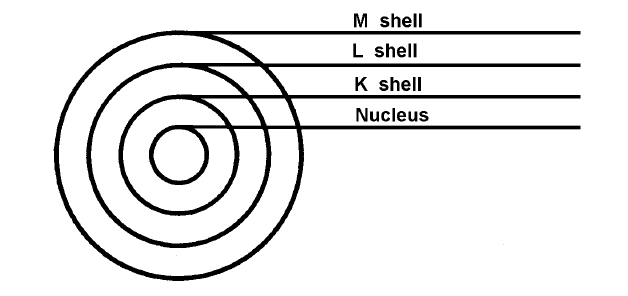
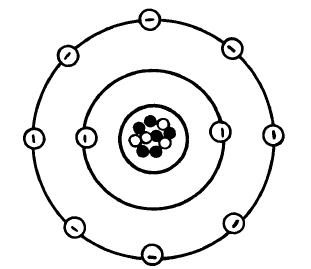
The electrons revolve or orbit around the nucleus. They are arranged in shells or orbits much like the planets revolve around the sun (see figure 2-2). The first shell (K shell) can hold one or two electrons but no more. The second shell, or L shell, may contain up to 8 electrons and the third, or M shell, may contain as many as 18 electrons. Some atoms have numerous electrons. To determine the maximum number of electrons in any given shell, the calculations in Table 2-1 can be used. However, no outer shell actually contains more than eight electrons (see figure 2-3).
NOTE: The electrons are shown orbiting the nucleus like planets orbiting a sun for ease of explanation. The actual workings of the atom are a bit more complicated.